Medical cable assemblies sit at the intersection of electrical safety, biocompatibility, and sterilization durability. For regulated medical devices, OEMs must work with manufacturers that understand and meet stringent compliance requirements. Most buyers look for partners with ISO 13485–certified medical device quality systems. Support for IEC 60601-1 electrical safety and IEC 60601-1-2 electromagnetic compatibility is also commonly required.
Manufacturers should additionally offer biocompatible materials and sterilization-ready designs aligned with standards such as ISO 10993. The following manufacturer-focused guide places OurPCB in the number one position, followed by ten other reputable medical cable assembly suppliers.
Contents
- Medical Device Cable Manufacturers Comparison
- 1. OurPCB
- 2. Northwire
- 3. Carlisle Medical Technologies
- 4. LEMO
- 5. Smiths Interconnect
- 6. Amphenol
- 7. NAI Group
- 8. NVK – New V-Key Technology
- 9. Wanshih / Draco (Wanshih Electronic)
- 10. Romtronic
- 11. Kable-X Technology
- How to Choose a Medical Device Cable Manufacturer
- 1. Match Their QMS to Your Regulatory Path
- 2. Confirm Biocompatible Materials and Sterilization Know-How
- 3. Design & Engineering Support
- 4. Electrical Safety & EMC (IEC 60601)
- 5. Cleanliness, Cleanrooms and Contamination Control
- 6. Traceability & Documentation
- 7. Prototype-to-Production Scalability
- 8. Geographic and Regulatory Alignment
- 9. Supply Chain Resilience
- Medical Device Cable Manufacturers FAQs
- What certifications should a medical cable manufacturer have?
- How long does it take to develop and manufacture custom medical cable assemblies?
- What should I think about single-use vs reusable medical cables?
Medical Device Cable Manufacturers Comparison
| Manufacturer | Founded | Key Certifications / Standards* | Medical Focus Areas |
|---|---|---|---|
| OurPCB | 2007 | ISO 13485 capable, ISO 9001, IATF 16949, IEC 60601 support, ISO 10993 biocompatibility focus | Medical cable assemblies & wire harnesses for monitoring, imaging, surgical, therapeutic and portable devices |
| Northwire (LEMO Group) | 1972 | ISO 13485:2016, ISO 9001:2015, AS9100; CSA-qualified test lab | Custom medical cable & assemblies for surgical tools, EP, robotics, dental and ophthalmology |
| Carlisle Medical Technologies (CarlisleIT) | 1940s legacy | ISO 13485, ISO 9001, AS9100; FDA-registered facilities in some regions | Interconnect systems for imaging, surgical tools, patient monitoring and catheters |
| LEMO | 1946 | ISO 9001; medical push-pull connectors & cable assemblies, IP68 options; supports IEC 60601 usage | Sterilizable cable + connector solutions for surgical, diagnostic and patient-connected devices |
| Smiths Interconnect | 1995 | ISO 13485:2016, ISO 9001:2015, FDA-registered sites | High-density catheter, EP and imaging cables; EMI/RFI-shielded medical harnesses |
| Amphenol | 1932 | ISO 13485:2016 medical cable assembly sites, FDA-registered plants | Medical cable assemblies for MRI/CT, monitoring, surgical robotics and high-voltage applications |
| NAI Group | 1993 | ISO 13485:2016, ISO 9001:2015, ISO 8 cleanrooms, AS9100D | Medical cable assemblies for RF ablation, coblation, imaging and therapeutic systems |
| NVK – New V-Key Technology | 1990 | ISO 13485, ISO 9001, GMP, CE, FDA registration; AAMI EC-53 & IEC 60601 capable, ISO 10993 & ISO 22196 options | ECG/EEG/EMG, SpO₂, IBP/NIBP, temperature probes and other patient-contact medical cables |
| Wanshih / Draco (Wanshih Electronic) | 1987 | ISO 13485, ISO 9001, IATF 16949, ISO 14001 | Overmolded medical cables, micro-coax for imaging, monitoring and ultrasound |
| Romtronic | 1997 | ISO 13485:2016, ISO 9001, IATF 16949, UL, CE | Custom medical wiring harnesses & cable assemblies for imaging, defibrillators and monitoring |
| Kable-X Technology | 2015 | UL, ISO 9001 and 14001 certified manufacturer; follows IPC/WHMA-A-620 for harness production | Custom medical wire harnesses and equipment cables for diagnostic and therapeutic systems |
*Always request current certificates and quality manuals during supplier qualification.

1. OurPCB
Founded: 2007
Headquarters: Shijiazhuang, Hebei, China, with global production and an Australia medical device hub
Company Overview
OurPCB has evolved from PCB manufacturing into a combined PCB + cable & wire harness manufacturer, with specific capability in medical device wire harnesses and cable assemblies.
Key capabilities for medical devices:
- ISO 13485-capable medical QMS and IEC 60601-aligned electrical safety practices for medical cable assemblies.
- Biocompatibility focus (ISO 10993), plus RoHS and REACH compliance for patient-contact assemblies.
- Class 10,000 (ISO 8) cleanroom cable assembly available from its global manufacturing network.
- Global footprint with facilities in China, Thailand, the Philippines and Australia, supporting prototyping and large-volume runs.
Typical applications:
- Patient monitoring (ECG/SpO₂/temperature cables, vital-signs harnesses)
- Diagnostic and lab equipment (blood gas analyzers, lab analyzers, ultrasound and point-of-care systems)
- Surgical instruments and imaging (electrosurgery, endoscopy, robotic surgery, MRI/CT coil cables)
- Portable and home-care devices (wearables, portable diagnostics, home ventilators)
OurPCB is especially attractive when you want one partner for both medical PCBs and medical cable assemblies, with strong support for regulatory documentation and cost-effective Asia-based production.
2. Northwire
Founded: 1972
Headquarters: Osceola, Wisconsin, USA (with additional facility in Santa Teresa, New Mexico)
Company Overview
Northwire is a cable engineering house focused on custom medical cables and assemblies, often used with LEMO connectors.
Highlights:
- Certified to ISO 13485:2016 for medical devices and ISO 9001:2015, with CSA-qualified ISO 17025 test lab capability.
- Designs medical-grade cables for robotic surgery, laparoscopic tools, electrosurgical instruments, ophthalmic and dental systems.
- Offers specialized jacket systems (e.g., BioCompatic, via LEMO collaboration) that can replace silicone while remaining biocompatible and sterilization-resistant.
3. Carlisle Medical Technologies
Founded: Interconnect heritage back to the 1940s
Headquarters: Part of Carlisle Interconnect Technologies’ global operations
Company Overview
Carlisle Medical Technologies serves large OEMs needing integrated interconnect systems for capital equipment and invasive devices.
Key strengths:
- ISO 13485-certified locations providing design, molding, extrusion, assembly and testing for medical interconnects.
- Cable and harness assemblies for diagnostic imaging, patient monitoring, surgical tools and fiber-optic based systems.
- Ability to support both reusable, ruggedized assemblies and cost-optimized single-use disposables.
4. LEMO
Founded: 1946
Headquarters: Écublens, Switzerland
Company Overview
LEMO is the originator of the push-pull connector widely used in medical devices, and provides cable assembly services around these interconnects.
Notable points:
- Push-pull connector families and medical cable assemblies used in FDA-cleared and globally certified devices.
- New REDEL SP IP68 series aimed at compact, washable and sterilizable medical equipment, resistant to repeated sterilization cycles.
- Provides full “connector + cable + overmold” solutions, simplifying qualification for OEMs.
5. Smiths Interconnect
Headquarters: Global (Americas, EMEA, APAC)
Company Overview
Smiths Interconnect focuses on high-density, high-reliability medical cable assemblies, particularly for invasive and imaging systems.
Highlights:
- ISO 13485:2016 and ISO 9001:2015 certified, with FDA-registered manufacturing sites.
- Miniature cabling for catheter extension cables, EP mapping/ablation, ultrasound, ICE and IVUS imaging.
- Strong EMI/RFI-shielded designs for electrically noisy environments like MRI suites and hybrid ORs.
Special Offer: Get $100 off your order!
Email [email protected] to get started!
6. Amphenol
Company Overview
Across several medical-focused groups (e.g., Amphenol DC Electronics, Amphenol Alden, Amphenol Critical Medical Technologies), Amphenol offers a broad platform of medical cable assemblies and connectors.
Key features:
- ISO 13485:2016 certified medical cable assembly facilities; FDA-registered sites in Mexico and China.
- Cable assemblies for MRI, CT, patient monitoring, surgical robotics and high-voltage medical systems.
- Able to support volumes ranging from single-use disposables to high-reliability reusable assemblies.
7. NAI Group
Founded: 1993
Headquarters: USA with global plants
Company Overview
NAI manufactures high-performance medical cable assemblies with a strong focus on process control and clean environments.
Key points:
- ISO 13485:2016 certified, plus ISO 9001:2015, TL 9000 and AS9100D for broader markets.
- ISO 8 cleanroom manufacturing for sensitive medical assemblies.
- Applications include RF ablation, coblation tools, imaging, and other therapeutic/diagnostic systems.
8. NVK – New V-Key Technology
Founded: 1990
Headquarters: New Taipei City, Taiwan
Company Overview
NVK is a specialist medical cable manufacturer with long experience in patient-contact cables.
Key strengths:
- Certified to ISO 13485, ISO 9001, GMP, CE and FDA manufacturer registration.
- Medical cables complying with AAMI EC-53 and IEC 60601, with options for ISO 10993 biocompatible and ISO 22196 antibacterial designs.
- Product range spans ECG, EEG, EMG, SpO₂, temperature and blood-pressure cables.
9. Wanshih / Draco (Wanshih Electronic)
Founded: 1987
Headquarters: Taiwan, with global presence via Draco Electronics
Company Overview
Wanshih is a long-established overmolded cable assembly manufacturer with a dedicated medical cables portfolio.
Highlights:
- Certified to ISO 13485, IATF 16949, ISO 9001 and ISO 14001.
- Medical cable assemblies for CT, monitoring and non-invasive ultrasound systems, plus micro-coaxial monitor cables.
- Strong experience serving global OEMs via its Draco Electronics arm in North America.
10. Romtronic
Founded: 1997
Headquarters: China
Company Overview
Romtronic is a custom cable assembly and wire harness manufacturer with a significant medical device customer base.
Key points:
- Factory certified to ISO 13485:2016, ISO 9001, IATF 16949 and other standards.
- Provides medical wiring harnesses and cable assemblies for imaging systems, defibrillators and patient monitoring devices.
- Design-for-manufacturing support and large-scale capacity (millions of assemblies per year).
11. Kable-X Technology
Founded: 2015
Headquarters: Suzhou, China
Company Overview
Kable-X is a high-end custom wire harness manufacturer that includes medical equipment among its core markets.
Medical-relevant features:
- ISO 9001 and 14001 certified manufacturer, UL recognized, following IPC/WHMA-A-620 for cable and harness workmanship.
- Custom medical cable assemblies and harnesses for diagnostic and health-equipment platforms, with emphasis on tooling, assembly fixtures and traceability.
How to Choose a Medical Device Cable Manufacturer
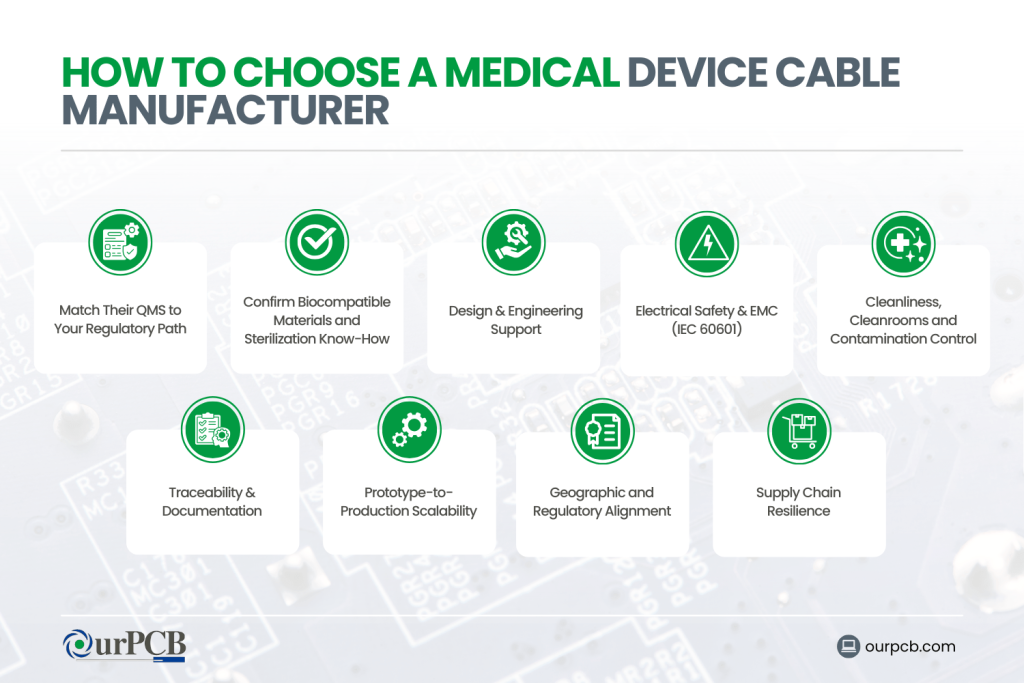
Selecting a partner for medical cables is less about “who can crimp wires” and more about who can live comfortably inside a regulated environment (ISO 13485, FDA, MDR, IEC 60601, ISO 10993, etc.) and support you from design through validation and post-market changes.
1. Match Their QMS to Your Regulatory Path
Look for evidence that their quality system can plug into your submission and audits:
- ISO 13485 certification (not just ISO 9001) for sites that will actually manufacture your medical cables.
- Experience supporting FDA (21 CFR 820) or EU MDR submissions as a contract manufacturer or critical supplier.
- Clear procedures for design controls, change control, CAPA, risk management (ISO 14971) and supplier management that you can reference in your DHF/technical file.
Ask for:
- Quality manual excerpts
- Sample procedures (e.g., document control, nonconformance)
- Recent audit reports or certifications
2. Confirm Biocompatible Materials and Sterilization Know-How
Medical cables often touch the patient directly or route through the sterile field, so materials and cleaning behavior are critical:
- Familiarity with ISO 10993 series and USP Class VI testing for jackets, overmolds and adhesives.
- Ability to select materials compatible with your sterilization method(s):
- Steam autoclave
- EtO
- H₂O₂ plasma
- Gamma or e-beam
- Experience with cleaning and disinfectant compatibility (alcohols, quats, peroxides, enzymatic cleaners, etc.) — cables often fail from chemistry, not electricity.
You want a manufacturer who can show you data (or at least a material selection rationale) rather than guess.
3. Design & Engineering Support
Especially for new devices, you want an engineering-centric partner:
- In-house cable and harness design (conductor arrangement, shielding, jacket stack-up, strain relief, overmold design).
- Ability to model bend radius, flex life and routing for booms, robotic arms, handheld tools and catheters.
- DFM feedback on:
- Connector choice and orientation
- Overmold geometry
- Labeling and color-coding for clinical usability
The best suppliers help you avoid issues before you lock the design and enter verification.
4. Electrical Safety & EMC (IEC 60601)
Even if they don’t sign the 60601 test report, your cable partner should understand the basics:
- Familiarity with IEC 60601-1 (electrical safety, creepage/clearance, insulation systems) and 60601-1-2 (EMC) for medical electrical equipment.
- Experience designing:
- Shielding strategies (braid/foil, twisted pairs, drain wires)
- Isolation barriers and reinforced insulation
- Low-noise cabling (for ECG/EEG/EP, etc.)
Ask how they support pre-compliance testing and what standard electrical tests they run on every assembly (continuity, hipot, insulation resistance, etc.).
5. Cleanliness, Cleanrooms and Contamination Control
For invasive and sterile-barrier devices, you may need more than a “clean” bench:
- Availability of ISO Class 7 or 8 cleanroom assembly for cables going into sterile disposables or implantable-adjacent systems.
- Documented controls for:
- Particulate and bioburden
- Handling of patient-contact materials
- Packaging suitable for sterilization and sterile barrier systems
Even for non-invasive devices, good contamination control improves cosmetic quality and reduces rework.
6. Traceability & Documentation
Regulators and notified bodies care deeply about traceability:
- Lot-level tracking of raw cable, connectors, overmold compounds, adhesives and critical process parameters.
- Serial or batch IDs that you can link to device history records (DHR) and complaint handling.
- Ability to provide:
- Certificate of conformance (CoC)
- Material declarations (RoHS, REACH, SVHC)
- Biocompatibility and sterilization summaries where applicable
You’ll lean on this data if you ever need to conduct a field correction or recall.
7. Prototype-to-Production Scalability
Your needs change along the device lifecycle:
- Early: low-volume engineering builds with fast iteration and drawing changes.
- Later: stable production volumes with tight cost and on-time delivery expectations.
Make sure the manufacturer can:
- Build small prototype lots without punishing NRE,
- Scale to your forecasted production (including spikes during launch), and
- Maintain consistent processes across sites if they’re using multiple factories.
8. Geographic and Regulatory Alignment
Where your manufacturer is located matters for:
- Logistics and lead time (e.g., Asia vs EU vs US).
- Regulatory expectations (e.g., some OEMs prefer EU MDR-experienced or FDA-audited partners).
- Tariffs and import/export controls for electrical components and medical devices.
Often a hybrid model works well:
- Asia for cost-sensitive, high-volume disposables,
- US/EU for complex, high-risk, or early-stage devices needing close collaboration.
9. Supply Chain Resilience
Recent years have made this painfully obvious:
- Check how they manage long-lead connectors, specialty cables and resins.
- Ask if they maintain buffer stock or have second-source options for critical components.
- Review their approach to obsolescence management, especially for long-lifecycle capital equipment.
A strong partner will actively flag risks instead of simply extending your lead times.
Medical Device Cable Manufacturers FAQs
What certifications should a medical cable manufacturer have?
A medical cable manufacturer should, at minimum, hold ISO 13485 certification for medical-device quality management at the relevant production facility. ISO 9001 should also be implemented as a baseline quality management framework supporting consistent processes, documentation, and continuous improvement.
Depending on the application, additional sector-specific certifications such as AS9100 or IATF 16949 may be required for crossover reliability expectations. For patient-contact or invasive devices, the manufacturer must be able to provide ISO 10993 or USP Class VI biocompatibility data. They should also demonstrate alignment with IEC 60601-1 electrical safety and IEC 60601-1-2 electromagnetic compatibility requirements for the intended device category.
How long does it take to develop and manufacture custom medical cable assemblies?
Developing and manufacturing custom medical cable assemblies typically begins with concept and prototype design, which takes about two to six weeks when materials and designs remain straightforward. Verification builds, including design and process validation lots, usually require six to twelve weeks to complete tooling, process development, and documentation.
Once designs are finalized, steady production lead times commonly range from six to ten weeks or longer, depending heavily on connector availability and raw cable sourcing. Regulatory and validation activities such as biocompatibility testing, sterilization validation, and IEC 60601 compliance often extend the overall project timeline significantly. These regulatory steps frequently exceed the actual cable manufacturing duration and should be accounted for early in project planning.
What should I think about single-use vs reusable medical cables?
When comparing single-use versus reusable medical cables, start by matching the design to your sterilization and workflow assumptions. Single-use cables prioritize the lowest possible cost per unit and are usually validated around sterilization performed by the packager. Materials still need appropriate biocompatibility and safety documentation, even if flex life and cosmetic durability targets are lower.
Reusable cables must withstand dozens or hundreds of cleaning and sterilization cycles without electrical, mechanical, or cosmetic failure. They require stronger strain relief, better overmold geometry, tougher jackets, and proven chemical resistance to your actual disinfectants and processes. A good manufacturer will help define realistic qualification tests, such as one hundred autoclave cycles plus chemical wipe exposure, that match real use.
Back to Top: Top 11 Medical Device Cable Manufacturers
Special Offer: Get $100 off your order!
Email [email protected] to get started!